63. Rates of water exchange in 2,2'-bipyridine
and 1,10-phenanthroline complexes of Co(II) and Mn(II)

The rates and activation parameters of water exchange at pH 3.0 have been determined using variable temperature 17O NMR for four Co(II) complexes and one Mn(II) complex, viz. [Co(bpy)(H2O)4]2+, [Co(bpy)2(H2O)2]2+, [Co(phen)- (H2O)4]2+, [Co(phen)2(H2O)2]2+, and [Mn(bpy)(H2O)4]2+. Substitution of 1,10-phenanthroline or 2,2'-bipyridyl for aquo ligands leads to an increase in the rate of exchange in the manganese complexes, from k 298 (1.8 ± 0.1) · 10 7 for [Mn(H2O)6]2+ to (7.2 ± 0.3) · 10 7 s -1 for [Mn(phen)2(H2O)2]2+ , whereas the trends are more complex for the cobalt complexes. We have used the new data in conjunction with literature data for similar complexes to analyse the impact of M-OH2 distance and degree of substitution.
Acharya, Shravan S.; Winther-Jensen, Bjorn; Spiccia, Leone; Ohlin, C. André; Rates of water exchange in 2,2'-bipyridine and 1,10-phenanthroline complexes of Co(II) and Mn(II) Aust. J. Chem. , Accepted. Link
62. 27Al MQMAS of the δ-Al13-Keggin

Abstract: One-dimensional 27Al, 23Na, Magic-Angle-Spinning (MAS) NMR and 27Al Multiple-Quantum Magic-Angle-Spinning NMR (MQMAS) measurements are reported for the δ-isomer of the Al13 Keggin structure at high spinning speed and 14.1 T field. Values for the CQ and η parameters are on the same scale as those seen in other isomers of the Al13 structure. Density functional theory (DFT) calculations are performed for comparison to the experimental fits using the B3PW91/6-31+G* and PBE0/6-31+G* levels of theory, with the Polarizable Continuum Model (PCM).
Pilgrim, Corey D.; Callahan, Joseph R.; Christopher A. Colla; Ohlin, C. André; Harris E., Mason; Casey, William H. 27Al MQMAS of the δ-Al13-Keggin Dalton Trans., 2017, 46, 2249-2254. Link
61. A Non-Aqueous Microwave Assisted
Protocol for Accessing Molybdovanadates
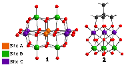
Abstract: We report a novel approach for the synthesis of heterohexa- and heterodecametalates via the use of non-aqueous, microwave assisted reaction conditions. The two novel molybdovanadates have been isolated and characterized in the solid and solution states using single-crystal X-ray diffraction, FT-IR, UV/Vis, multinuclear NMR, and ESI-MS. The relative stabilities of the possible structural isomers were probed using DFT calculations for both polyoxometalate systems
Spillane, Samuel; Sharma, Rupali; Zavras, Athanasios; Mulder, Roger; Ohlin, C. André; Goerigk, Lars; Best, Stephen P.; O'Hair, Richard A. J.; Ritchie, Chris A Non-Aqueous Microwave Assisted Protocol for Accessing Molybdovanadates Angew. Chem. Int. Ed., Accepted. Link
60. Heavier Group 13 Metal(I) Heterocycles
Stabilized by Sterically Demanding Diiminophosphinates: A Structurally Characterized Monomer-Dimer Pair For Gallium
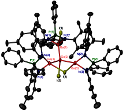
Abstract: We have synthesized and characterized the monomeric diiminophosphinate-stabilized group 13 metal(I) complexes [DipLE:], DipL = Ph2P(NDip)2, Dip = 2,6-iPr2C6H3; E = Ga (1), In (2) and Tl (3). In addition, we structurally characterized the dimeric complex [(DipLGa)2], 12. Similar synthetic attempts using MesL = Ph2P(NMes)2, Mes = 2,4,6-Me3C6H2 afforded product mixtures from which the mixed oxidation state species [(MesL)3Ga4I3] 4 was isolated. [DipLGa:] 1 is converted with dry air to the gallium(III) oxide species [(DipLGaO)2] 5. Density Functional Theory studies on [DipLE:] and [(DipLE)2], E = Al-Tl, shed light on the bonding in these compounds and show that the newly formed E-E bonding interactions can be described as weak single σ-bond with no significant π-bonding contribution for E = Al, Ga. A large contribution to the dimer binding enthalpies results from London dispersion forces.
Hawley, Andrew L.; Ohlin, C. André; Fohlmeister, Lea; Stasch, Andreas Heavier Group 13 Metal(I) Heterocycles Stabilized by Sterically Demanding Diiminophosphinates: A Structurally Characterized Monomer-Dimer Pair For Gallium Chem. Eur. J., 2017, 23(2), 447-455. Link
59. Solid-state 27Al NMR spectroscopy of the
γ-Al13 Keggin containing Al coordinated by a terminal hydroxyl ligand

Abstract: We report solid-state 27Al NMR spectroscopic results for the sulfate salt of the γ-Al13 Keggin cluster, γ-[AlO4Al12(OH)25(OH2)114]3[H2O]14 , that provide a spectroscopic signature for partial hydrolysis of this Keggin-type cluster. In 27Al multiple-quantum (MQ) MAS NMR spectra, all thirteen Al positions of the cluster are at least partially resolved and assigned with the aid of DFT calculations of the 27 Al electric field gradients. The isotropic chemical shift of the single tetrahedral site, 75.7 ppm, is nearly identical to 36 that reported for solutions from which the cluster crystallizes. Reflecting broadly similar coordination environments, the octahedral Al show mostly small variations in chemical shift (+7 to +11 ppm) and quadrupolar coupling constant (Cq; 6-7.5 MHz), except for one resonance that exhibits a much smaller Cq and another site with a larger value. DFT calculations show that deprotonation of a terminal water ligand, to form an η-OH group, causes a large reduction in the 27Al Cq, allowing assignment of a distinct, narrow peak for octahedral Al to this hydroxyl-terminated site. This result suggests a relationship between octahedral 27Al NMR linewidth and hydrolysis for solids prepared from Keggin-type clusters.
Phillips, Brian L.; Ohlin, C. André; Vaughn, John; Woerner, William; Smart, Scott; Pan, Long Solid-state 27Al NMR spectroscopy of the γ-Al13 Keggin containing Al coordinated by a terminal hydroxyl ligand Inorg. Chem., 2016, 55(23), 12270-12280. Link
58. Characterization of decavanadate
and decaniobate solutions by Raman spectroscopy
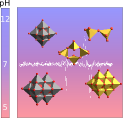
Abstract: The decaniobate ion, (Nb10=[Nb10O28]6-) being isoelectronic and isostructural with the decavanadate ion (V10=[V10O28]6-), but chemically and electrochemically more inert, has been useful in advancing the understanding of V10 toxicology and pharmacological activities. In the present study, the solution chemistry of Nb10 and V10 between pH 4 and 12 is studied by Raman spectroscopy. The Raman spectra of V10 show that this vanadate species dominates up to pH 6.45 whereas it remains detectable until pH 8.59, which is an important range for biochemistry. Similarly, Nb10 is present between pH 5.49 and 9.90 and this species remains detectable in solution up to pH 10.80. V10 dissociates at most pH values into smaller tetrahedral vanadate oligomers such as V1 and V2, whereas Nb10 dissociates into Nb6 in mildly (10>pH>7.6) or high alkaline conditions. Solutions of V10 and Nb10 are both kinetically stable at basic pH conditions for at least two weeks and moderate temperature. The Raman method provides a means of establishing speciation in the difficult niobate system and these findings have important consequences for toxicology activities and pharmacological applications of vanadate and niobate polyoxometalates.
Aureliano, Manuel; Ohlin, C. André; Viera, Michele O; Marques, M. Paula M.; Casey, William H.; Batista de Carvalho, Luis A. E. Characterization of decavanadate and decaniobate solutions by Raman spectroscopy Dalton Trans., 2016, 45, 7391-7399. Link
57. Predicting 17O NMR chemical shifts of polyoxometalates
using density functional theory
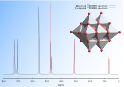
Abstract: We have investigated the computation of 17O NMR chemical shifts of a wide range of polyoxometalates using density functional theory. The effects of basis set and exchange correlation functional are explored, and whereas pure DFT functionals generally predict the chemical shifts of terminal oxygen sites quite well, hybrid functionals are required for the prediction of accurate chemical shifts in conjunction with linear regression. Using PBE0/def2-tzvp//PBE0/cc-pvtz(H-Ar), lanl2dz(K-) we have computed the chemical shifts of 37 polyoxometalates, corresponding to 209 17O NMR signals. We also show that at this level of theory the protonation-induced pH dependence of the chemical shift of the triprotic hexaniobate Lindqvist anion, [HxNb6O19](8-x)-, can be reproduced, which suggests that hypotheses regarding loci of protonation can be confidently tested.
Sharma, Rupali; Zhang, Jie; Ohlin, C. André Predicting 17O NMR chemical shifts of polyoxometalates using density functional theory Phys. Chem. Chem. Phys., 2016, 18, 8235-8241. Link
56. Mechanistic studies of the photo-electrochemical
hydrogen evolution reaction on poly(2,2'-bithiophene)
Abstract: The realisation of poly(2,2'-bithiophene) (PBTh) as an effective photo-electrocatalyst for the hydrogen evolution reaction is a novel discovery [Ng et al., Int. J. Hydrogen Energy, 2014, 39, 18230], however, the underlying mechanism of this catalysis remains unknown. In this article, studies using electrochemical, photo-electrochemical, Raman spectroscopy and computational modelling are undertaken to shed some light on the mechanistic process. From these studies, a compelling reaction scheme is proposed involving the protonation of the PBTh chain via the sulphur atom and subsequent intersystem crossing to a long-lived triplet state for the reaction to form H2. This suggested mechanism is tentative but cohesively integrates all experimental and computational findings. Importantly, these insights into the PBTh system form an important mechanistic milestone study and will help inspire new developments and applications for polythiophenes and conducting polymers.
Ng, Chun Hin; Ohlin, C. André; Qiu, Siyao, Sun, Chenghua, Winther-Jensen, Bjorn
Mechanistic studies of the photo-electrochemical
hydrogen evolution reaction on poly(2,2'-bithiophene
Cat. Sci. Techn., 2016, 6, 3252-3262. Link
