14. Distinctly different reactivities of two similar
polyoxoniobates with hydrogen peroxide
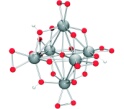
Abstract Peroxoniobate species are found whenever niobium oxides are employed as photocatalysts or used to sequester radionuclides. The reactions of [Nb6O19]8- and [Nb 10O28]6- with hydrogen peroxide each behave differently, and the first example of a peroxopolyoxoniobate species, [N(CH3)4]5[H3Nb6O13 (O(I)2)6] . 9.5 H2O, was structurally characterized (see picture; Nb gray, O red, H white).
Ohlin, C. André,[1,2] Villa, Eric M.,[1,2] Fettinger, James C.,[1] Casey, William H. [1,2] Distinctly different reactivities of two similar polyoxoniobates with hydrogen peroxide, Angew. Chem. Int. Ed., 2008,47(43), 8251-8254.
1. Department of Chemistry, University of California, Davis, California
2. Department of Geology, University of California, Davis, California
13. Adding reactivity to structure
- reaction dynamics in a nanometer-size oxide ion in water
Abstract We examine oxygen-isotope exchanges in a nanometer-size oxide molecule in water and, separately, both its rates of dissociation and molecular products. This molecule, the decaniobate ion ([HxNb10O28](6-x)-), is at the same size scale as geochemically interesting features on minerals, such as surface polymers and kink sites on growth steps, although it is structurally quite dissimilar. Unlike mineral surface structures, however, we have complete confidence in the aqueous structure of this molecule and it yields a clear spectroscopic signature as it reacts. We thus can follow proton-enhanced isotope exchanges and base-induced dissociation in unprecedented detail and clarity. The results are surprising and require new thinking about geochemical reactions at the molecular scale. For example, base-induced dissociation of the molecule, which is unprotonated, causes rates of oxygen-isotope exchanges of all structural oxygens to accelerate dramatically. Similarly, protonation of the molecule causes sets of oxygens to react, although protonation is limited. In general, all reactions are via concerted motions of many atoms and the reactivities vary as though the entire structure was responding to changes in solution composition. The site reactivities could not be inferred from the stable structure of the decaniobate molecule because so much of the structure is involved in each exchange event. Thus, computational models must be structurally faithful to an extraordinary degree, and inherently dynamic, or they will miss the essential chemistry.
Villa, Eric M.,[1,2] Ohlin, C. André,[1,2] Balogh, Edina,[1,2] Anderson, Travis M.,[3] Nyman, May D.,[3] Casey, William H. [1,2] Adding reactivity to structure - reaction dynamics in a nanometer-size oxide ion in water, Am. J. Sci., 2008, 308, 942-953.Link
1. Department of Chemistry, University of California, Davis, California
2. Department of Geology, University of California, Davis, California
3. Geochemistry Division, Sandia National Laboratories, Albuquerque, New Mexico
12. The [Ti12Nb6O44]-10 ion - A new type of
polyoxometalate
structure
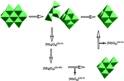
Abstract An extraordinarily useful cluster to detail reaction pathways in water might have been obtained in form of [N(CH3)4]10[Ti12Nb6O44], a polyoxometalate with a central cavity. This compound is soluble in a range of solvents and has been characterized by ESI mass spectrometry and X-ray crystallography (see picture).
Ohlin, C.André,[1,2] Villa, Eric M.,[1,2] Fettinger, James C.,[1] Casey, William H. [1,2] The [Ti12Nb6O44 ]-10 ion - A new type of polyoxometalate structure, Angew. Chem. Int. Ed. 2008 , 47(30), 5634-5646.
1. Department of Chemistry, University of California, Davis, California
2. Department of Geology, University of California, Davis, California
11. Reaction dynamics of the decaniobate ion
[HxNb10O28](6-x)-
in water
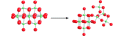
Abstract The nanometer-size title cluster is unique in that it reacts slowly enough in water that one can simultaneously observe steady-state oxygen-isotope exchanges and dissociation pathways (see scheme; O red, Nb green), leading to conceptual advances in both geochemistry and polyoxometalate chemistry.
Villa, Eric M.,[1,2] Ohlin, C. André,[1,2] Balogh, Edina,[1,2] Anderson, Travis M.,[3] Nyman, May D.,[3] Casey, William H. [1,2] Reaction dynamics of the decaniobate ion [HxNb10O28](6-x)- in water, Angew. Chem. Int. Ed., 2008, 47(26), 4844-4846.
1. Department of Chemistry, University of California, Davis, California
2. Department of Geology, University of California, Davis, California
3. Geochemistry Division, Sandia National Laboratories, Albuquerque, New Mexico
10. Cell directional migration and
oriented division on three-dimensional laser-induced periodic surface structures on polystyrene

Abstract The extracellular matrix in animal tissues usually provides a three-dimensional structural support to cells in addition to performing various other important functions. In the present study, wavy submicrometer laser-irradiated periodic surface structures (LIPSS) were produced on a smooth polystyrene film by polarized laser irradiation with a wavelength of 266 nm. Rat C6 glioma cells exhibited directional migration and oriented division on laser-irradiated polystyrene, which was parallel to the direction of LIPSS. However, rat C6 glioma cells on smooth polystyrene moved in a three-step invasion cycle, with faster migration speed than that on laser-irradiated polystyrene. In addition, focal adhesions examined by immunostaining focal adhesion kinase in human epithelial carcinoma HeLa cells were punctuated on smooth polystyrene, whereas dash-like on laser-irradiated polystyrene. We hypothesized that LIPSS on laser-irradiated polystyrene acted as an anisotropic and persistent mechanical stimulus to guide cell anisotropic spreading, migration and division through focal adhesions.
Wang, Xuefeng;[1,2] Ohlin, C. André;[3] Lu, Qinghua;[1] Hu, Jun [2] "Cell directional migration and oriented division on three-dimensional laser-induced periodic surface structures on polystyrene", Biomaterials, 2008, 29(13), 2049-2059. Link.
1. School of Chemistry and Chemical Technology, Shanghai Jiao Tong University, Shanghai
2. School of Life Sciences and Biotechnology, Shanghai Jiao Tong University, Shanghai
3. Department of Chemistry, University of California at Davis, One Shields Avenue, Davis, CA
